
Adalimumab? Brodalumab? Abrocitinib? Have you ever wondered: is there an easy way to remember not only what class each drug belongs to, but also how to pronounce these generic names? I must admit, I commonly slaughter the generic names of biologic drugs. I usually add a vowel, a consonant or syllable which isn’t present and make the name ten times longer and much more complicated to pronounce. But I have learned a few tricks along the way, which I am eager to share with you.
Let’s first talk about pronunciation.
It is really simple and should just take us all back to elementary school. The pronunciation of these medications is a case of sounding out each syllable. Brodalumab is pronounced: Brod-a-l-u-mab. When this drug was first launched, I added all kinds of other components and hilariously called it Broadulibumab! One of the things I find interesting about these medications is how each biologic receives its name and why. Believe it or not, there is a method to the madness! The World Health Organization (WHO) has taken the lead on assigning what is known as the INN or International Nonproprietary Name(generic name)tothe world’s medication supply.
The naming of a biologic medication must follow a few simple guidelines:
1. Differentiate the medication from another for patient safety,
2. Allow for manufacturer tracking
3. Help those prescribing and dispensing by implementing a class specific suffix.
These guidelines seem common sense and are not new to us. Most of us are familiar with common suffix for ARBs: -sartan and -pril for ACEi. Biologics are no different. Monoclonal antibodies end with-mab, while Janis Kinase (JAK) inhibitors have the suffix-citinib. But where does the rest of the name come from? At one time, medications were named based on their chemical structure. We can all see theproblem with this process, as multiple medications having similar chemical structures. Let’s take a moment to look at the breakdown of the components which make up the name of amonoclonal antibody. The name is comprised of a prefix (chosen at random), substem A (molecule target), substem B (source species) and thesuffix, -mab.Vowels are added along the way to simply help with pronunciation.
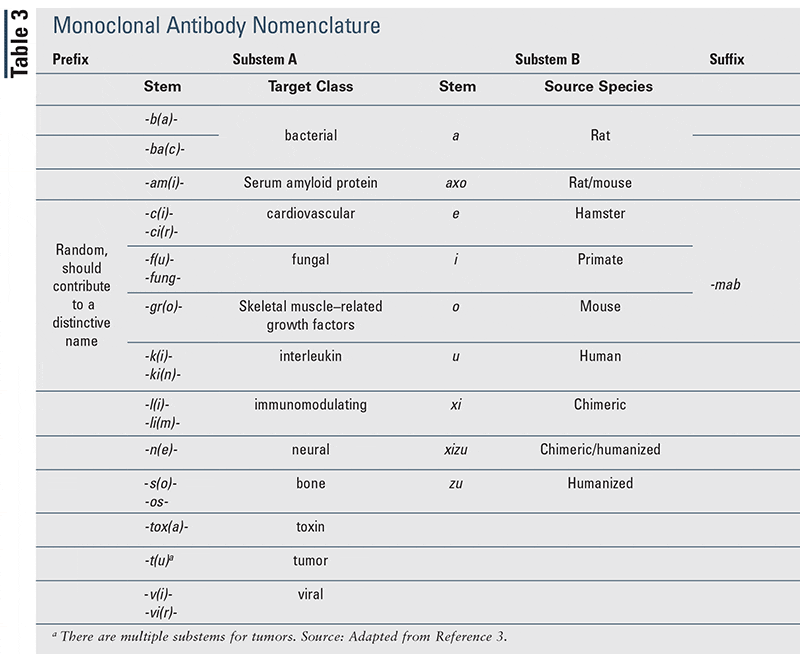
If we look at adalimumab using the guidelines above, here is what we know: 1. Ad: a random prefix2. -a: vowel added to help with pronounciation3. -lim (substem A): target -immunomodulator 4. -u(substem B): source species –humanized antibody5. -mab(suffix): monoclonal antibody With the release of biosimilars, the pronunciation may become a little more challenging. The FDA is requiring the addition of yet another four-letter suffix, devoid of meaning, to the end of each biologic, to indicate a separate, but biologically similar, medication. Hence a biosimilar for adalimumab will be something likeadalimumab-afzb. Fortunately for me, I can just pronounce each letter of the new suffix and not try to make a whole new word out of this name!
Register for a Skin Bones CME Conference Today!
Hit the reset button while earning continuing medical education credits at our CME conferences, where you can travel to a vacation destination; earn CME credits with like-minded nurse practitioners, physician assistants, and physicians; and ‘unplug’ while enjoying a new locale! Check out our upcoming Skin, Bones, Hearts & Private Parts 2023 CME Conferences and 2024 CME Conferences! At every event, the best of the medical community gathers to earn CME credits, network, and gain knowledge on dermatology, orthopedics, cardiology and emergency medicine, women’s health, pain management and pharmacology, diabetes, ER, and mental health. On-line CME courses and Virtual CME are also available so you have the option of earning CME credits online.
References:
1. Naming of Biological Products; Gordon L Peters, PharmD; Erin K. Hennessey, PharmD;
US Pharm. 2020;45(6) 33-36
Published June 18, 2020
2. How Do Drugs Get Named?Gail Karet, PhD; AMA Journal of Ethics; History of Medicine Aug 2019